Does Dysport Have Long-Term Effects?
Understanding Dysport
Dysport is a popular injectable treatment used to reduce the appearance of fine lines and wrinkles by temporarily relaxing the muscles that cause them. It works similarly to Botox and is commonly used to treat areas such as frown lines, forehead wrinkles, and crow’s feet. While Dysport is known for its short-term effectiveness, many patients wonder whether it has any long-term effects, either positive or negative.
How Dysport Works
Dysport contains botulinum toxin type A, which blocks nerve signals to the muscles, temporarily reducing their activity. This allows the overlying skin to smooth out, minimizing the appearance of wrinkles. The effects of Dysport typically last 3 to 4 months, after which muscle activity gradually returns to normal, and wrinkles may reappear if no further treatments are administered.
Are There Any Long-Term Effects of Dysport?
Dysport is designed to provide temporary results, and there are no permanent changes to the muscles or skin after the product wears off. However, with repeated use over an extended period, some subtle long-term effects can occur:
- Muscle Weakening: Repeated Dysport injections can lead to temporary weakening of the targeted muscles. This weakening is not harmful and typically reverses once the treatments are stopped.
- Reduced Wrinkle Formation: Regular use of Dysport can prevent dynamic wrinkles (those caused by muscle movements) from becoming static wrinkles (permanent lines that appear even at rest). This can result in smoother skin in the long run.
- Prolonged Results Over Time: Some patients report that the effects of Dysport last longer with consistent use, potentially allowing for longer intervals between treatments.
Does Dysport Cause Any Negative Long-Term Effects?
Dysport has been used safely for many years, and most studies do not show any adverse long-term effects when administered correctly. However, some potential risks include:
- Resistance to Treatment: Over time, some patients may develop resistance to Dysport due to the formation of antibodies against the botulinum toxin. This can make the treatment less effective.
- Muscle Imbalance: If Dysport is not administered evenly or if surrounding muscles overcompensate, it can lead to temporary muscle imbalance or asymmetry.
To minimize these risks, it’s crucial to work with an experienced provider who understands facial anatomy and has a thorough understanding of Dysport’s effects.
Can Dysport Improve Skin Quality Long-Term?
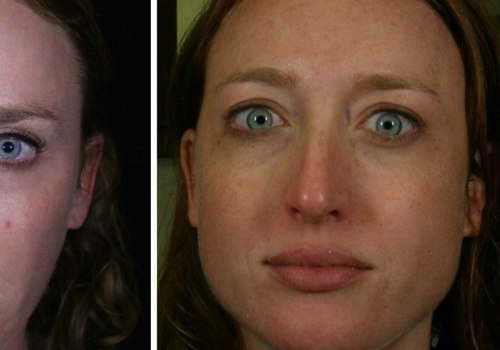
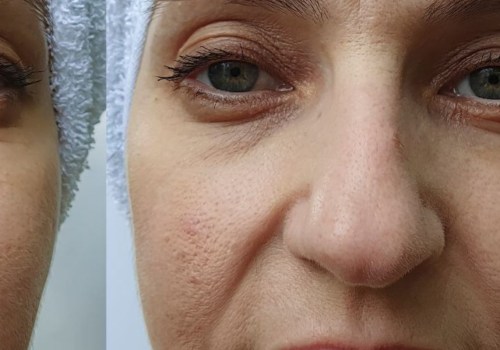
Some studies suggest that regular Dysport use may have positive long-term effects on skin quality. By relaxing facial muscles and preventing repetitive movements that cause wrinkles, Dysport may help maintain smoother, firmer skin over time. While these effects are not permanent, they can contribute to a more youthful appearance with continued treatment.
Conclusion
Dysport is a safe and effective treatment for temporarily reducing the appearance of wrinkles. While it does not cause permanent changes, consistent use over time can lead to subtle long-term benefits, such as reduced wrinkle formation and prolonged results. If you’re considering Dysport, consult a qualified provider to discuss the potential long-term effects and create a personalized treatment plan that suits your needs.